Research Summary
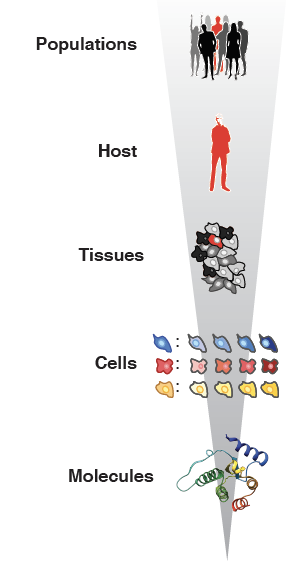
RNA viruses are a major threat to human health and well-being. They also provide a fascinating window into cell biology and immunology, as they interact intimately with their hosts at both cellular and systemic levels. The constant interplay between viruses trying to establish infection and the host trying to control them presents an ideal system to study evolution in action. In the lab we are interested in understanding these diverse but interconnected aspects of RNA virus biology.
- Virus Evolution
- Live Attenuated Vaccines
- Self-replicating RNAs Therapies and Vaccines
- Identifying Novel Antiviral Targets
Virus Evolution
Viral infections are marked by continuous evolution and adaptation to ever-changing environments. To thrive, viruses must successfully replicate across diverse tissues that are constantly altered by the infection itself. Studying how viruses adapt to these challenges not only deepens our understanding of viral pathogenesis but also informs the development of innovative antiviral therapies and live-attenuated vaccines. To advance this knowledge, we employ experimental evolution models and create computational tools to analyze the mutation profiles of RNA viruses, shedding light on their adaptability and mechanisms of survival.
- Acevedo, A., Brodsky, L., Andino, R., 2014. Mutational and fitness landscapes of an RNA virus revealed through population sequencing. Nature 505, 686–690. https://doi.org/10.1038/nature12861
- Stern, A., Bianco, S., Yeh, M.T., Wright, C., Butcher, K., Tang, C., Nielsen, R., Andino, R., 2014. Costs and Benefits of Mutational Robustness in RNA Viruses. Cell Rep. 8, 1026–1036. https://doi.org/10.1016/j.celrep.2014.07.011
- Stern, A., Yeh, M.T., Zinger, T., Smith, M., Wright, C., Ling, G., Nielsen, R., Macadam, A., Andino, R., 2017. The Evolutionary Pathway to Virulence of an RNA Virus. Cell 169, 35-46.e19. https://doi.org/10.1016/j.cell.2017.03.013
Live Attenuated Vaccines
Viruses have triggered numerous outbreaks across the globe, prompting the development of various rapid response measures. Among these are inactivated vaccines, including killed viruses, mRNA-based vaccines, and subunit vaccines. While these approaches have proven effective in reducing the severity of diseases, they often fall short in preventing the spread of the virus, as demonstrated during the SARS-CoV-2 pandemic. In light of these limitations, our efforts are focused on advancing the development of live attenuated vaccines, which hold greater potential for curbing virus transmission.
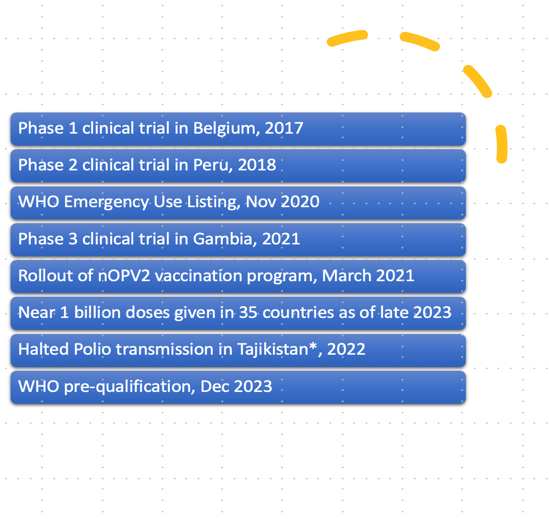
Leveraging our understanding of virus replication and evolution, we have developed innovative poliovirus vaccine strains aimed at eradicating global poliomyelitis. While live-attenuated polio vaccines, such as the Sabin strains, have significantly reduced polio cases over the past few decades, vaccine-derived polioviruses (VDPVs) remain a major obstacle to complete eradication. To address this challenge, we analyzed poliovirus evolution to create genetically stable, safer, and more immunogenic novel oral polio vaccines (nOPVs) for all three poliovirus types. These advancements represent a critical step toward overcoming the final barriers to a polio-free world (Konopka-Anstadt et al., 2020; Stern et al., 2017; Yeh et al., 2023, 2020). Among them, nOPV1 and nOPV3 are being tested in phase 2 clinical trials. nOPV2 has been granted WHO pre-qualification and over 1 billion doses have been administered in over 35 countries since March 2021. Using lessons learned from nOPVs, we target other enteroviruses to generate promising vaccine strains with manipulated tissue tropism.
- Konopka-Anstadt, J.L., Campagnoli, R., Vincent, A., Shaw, J., Wei, L., Wynn, N.T., Smithee, S.E., Bujaki, E., Yeh, M.T., Laassri, M., Zagorodnyaya, T., Weiner, A.J., Chumakov, K., Andino, R., Macadam, A., Kew, O., Burns, C.C., 2020. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. npj Vaccines 5, 26. https://doi.org/10.1038/s41541-020-0176-7
- Yeh, M.T., Bujaki, E., Dolan, P.T., Smith, M., Wahid, R., Konz, J., Weiner, A.J., Bandyopadhyay, A.S., Damme, P.V., Coster, I.D., Revets, H., Macadam, A., Andino, R., 2020. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 27, 736-751.e8. https://doi.org/10.1016/j.chom.2020.04.003
- Yeh, M.T., Smith, M., Carlyle, S., Konopka-Anstadt, J.L., Burns, C.C., Konz, J., Andino, R., Macadam, A., 2023. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature 619, 135–142. https://doi.org/10.1038/s41586-023-06212-3
- Stern, A., Yeh, M.T., Zinger, T., Smith, M., Wright, C., Ling, G., Nielsen, R., Macadam, A., Andino, R., 2017. The Evolutionary Pathway to Virulence of an RNA Virus. Cell 169, 35-46.e19. https://doi.org/10.1016/j.cell.2017.03.013
Self-Replicating RNAs Therapies and Vaccines
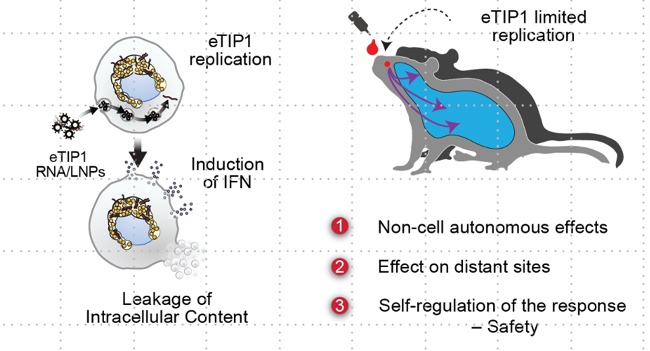
We have developed a self-replicating RNA (replicon) derived from live-attenuated poliovirus vaccine strains, designed to provide pre- and post-exposure protection against respiratory infections. Administered via lipid nanoparticles like mRNA vaccines, enterovirus replicons activate innate immune responses and enhance adaptive immunity, offering broad-spectrum protection against respiratory viruses such as rhinoviruses, influenza, and SARS-CoV-2 (Shirogane et al., 2021; Xiao et al., 2021). By mimicking natural infection, it generates a regulated antiviral response and long-term immunity. Our goal is to advance this strategy to clinical trials by optimizing delivery, formulation, and safety, addressing mucosal infections and emerging viral threats.
- Shirogane, Y., Rousseau, E., Voznica, J., Xiao, Y., Su, W., Catching, A., Whitfield, Z.J., Rouzine, I.M., Bianco, S., Andino, R., 2021. Experimental and mathematical insights on the interactions between poliovirus and a defective interfering genome. PLoS Pathog. 17, e1009277. https://doi.org/10.1371/journal.ppat.1009277
- Xiao, Y., Lidsky, P.V., Shirogane, Y., Aviner, R., Wu, C.-T., Li, W., Zheng, W., Talbot, D., Catching, A., Doitsh, G., Su, W., Gekko, C.E., Nayak, A., Ernst, J.D., Brodsky, L., Brodsky, E., Rousseau, E., Capponi, S., Bianco, S., Nakamura, R., Jackson, P.K., Frydman, J., Andino, R., 2021. A defective viral genome strategy elicits broad protective immunity against respiratory viruses. Cell 184, 6037-6051.e14. https://doi.org/10.1016/j.cell.2021.11.023
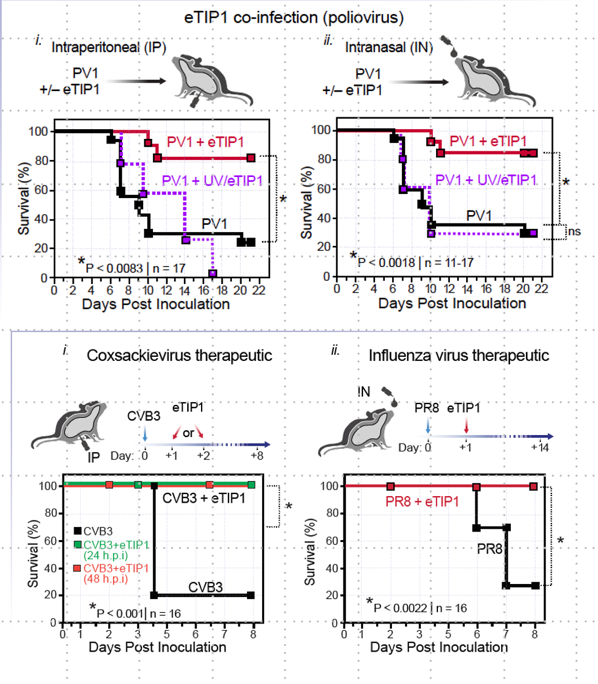
Strategies to identify Novel Antiviral Targets in Conserved Domains within Enterovirus Proteins and RNAs
This project focuses on developing antivirals targeting conserved RNA and protein interactions in viruses. Using the innovative CirSeq sequencing platform (Acevedo et al., 2014) , we identify invariant regions in viral genomes, resistant to mutations. Combining structural data and deep learning, we design drugs to interact with these conserved domains. The efficacy of these drugs is validated through biochemical assays, cell studies, and animal models, aiming to discover broad-spectrum therapies against enteroviruses (Barnes et al., 2008; Gitlin et al., 2005; Vignuzzi et al., 2008).
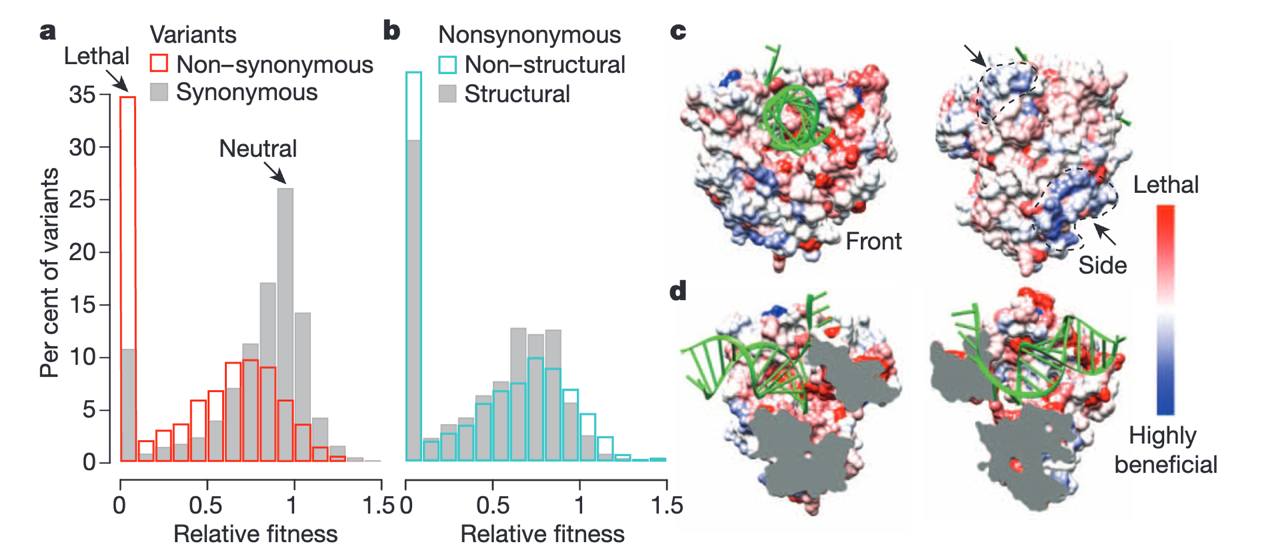
- Acevedo, A., Brodsky, L., Andino, R., 2014. Mutational and fitness landscapes of an RNA virus revealed through population sequencing. Nature 505, 686–690. https://doi.org/10.1038/nature12861
- Barnes, D., Kunitomi, M., Vignuzzi, M., Saksela, K., Andino, R., 2008. Harnessing Endogenous miRNAs to Control Virus Tissue Tropism as a Strategy for Developing Attenuated Virus Vaccines. Cell Host Microbe 4, 239–248. https://doi.org/10.1016/j.chom.2008.08.003
- Gitlin, L., Stone, J.K., Andino, R., 2005. Poliovirus Escape from RNA Interference: Short Interfering RNA-Target Recognition and Implications for Therapeutic Approaches. J. Virol. 79, 1027–1035. https://doi.org/10.1128/jvi.79.2.1027-1035.2005
- Vignuzzi, M., Wendt, E., Andino, R., 2008. Engineering attenuated virus vaccines by controlling replication fidelity. Nature medicine 14, 154–161. https://doi.org/10.1038/nm1726

